September 5, 2024
Tesofensine Peptide In Midlothian, Va
Negative Events
Why was tesofensine terminated?
Tesofensine was originally investigated for the therapy of Alzheimer''s illness and Parkinson''s illness, and was consequently gone down from development for these applications after very early trial results showed minimal effectiveness for therapy of these conditions.
What Is Tesofensine Peptide?
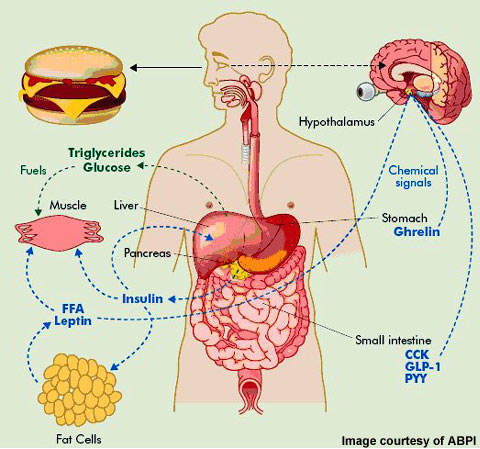
Our information is the initial to demonstrate that tesofensine directly targets LH feeding circuits, specifically silencing a part of GABAergic nerve cells, and turning on a still unidentified cell kind (perhaps a subset of glutamatergic neurons). It paves the way to reveal much better methods to enhance the healing effects of tesofensine and perhaps for other cravings suppressants. After demonstrating the anorexigenic impacts of tesofensine in lean Vgat-ChR2 mice, we intended to duplicate our searchings for in obese Vgat-IRES-cre mice.- Having these 3 natural chemicals prevented from being reabsorbed by the central nerve system causes the body feeling less starving.
- Almost a decade after obesity was categorized as a condition, leptin wasdiscovered and the idea of weight problems being a chronic, physiologically controlleddisease started to get traction [2]
- The pituitary gland hinges on hypothalamic signals that are regularly disrupted from hypothalamic damage, that influences secretion of growth hormonal agent, gonadotropins, adrenocorticotrophic hormonal agent (ACTH) and thyroid stimulating hormonal agent (TSH).
- . The systems of action of glucagon-like peptide-1 agonists and co-agonists, diabetes medications being examined for weight-loss, and medicines acting upon the central nervous system along with peripherally are evaluated.
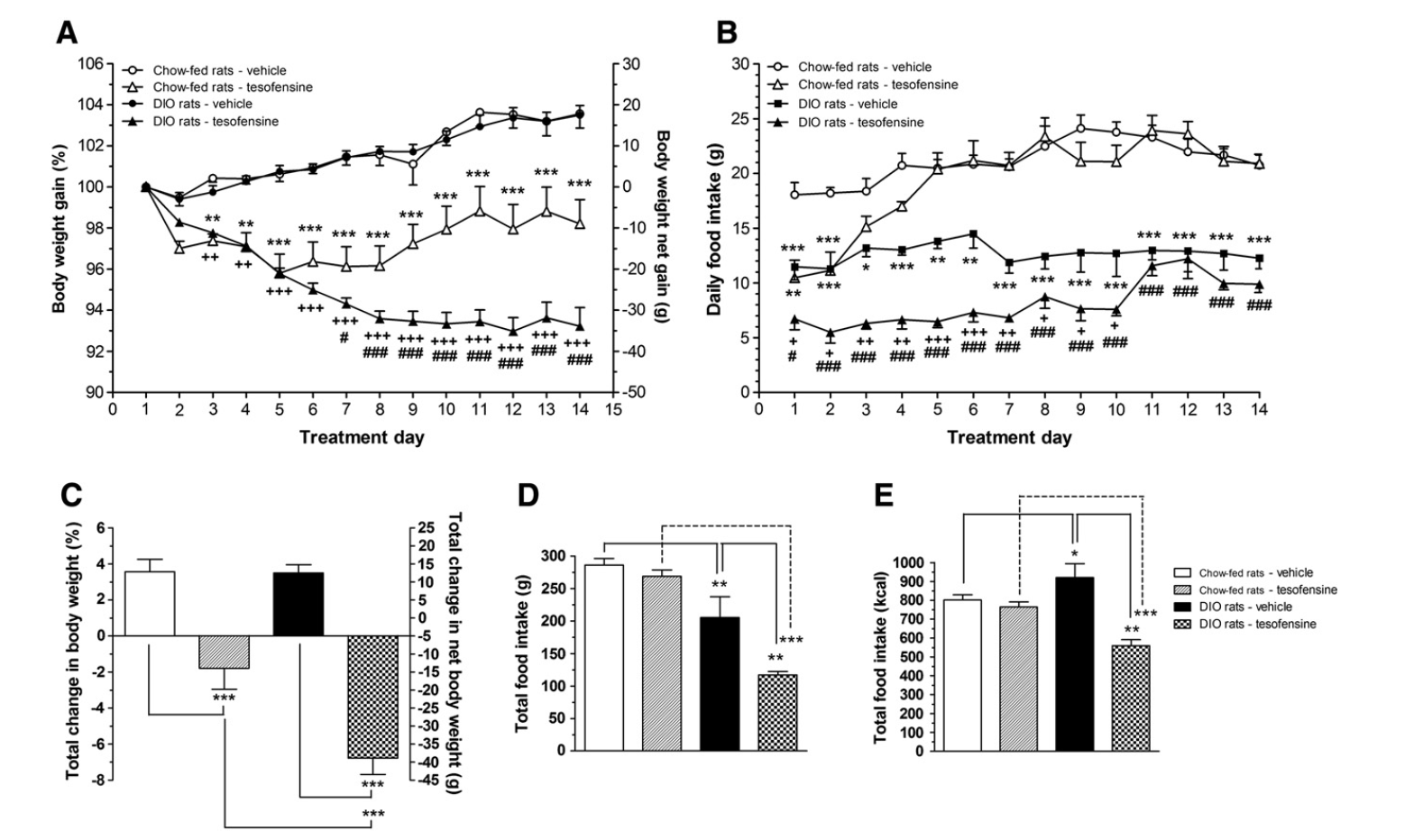
- Scientific researches and study demonstrate the efficacy of tesofensine in the domain name of weight reduction and excessive weight management.
- Upper panel shows the variety of tests, and the reduced panel the appropriate efficiency throughout the standard, tesofensine treatment, and post-tesofensine days.
Safety And Security Elements
In the last century, the pharmacological administration of weight problems has included amphetamines, thyroid hormones, dinitrophenol and different drug Get more info mixes (rainbow pills) that were taken out quickly after regulative authorization as a result of major negative effects34 (Table 1). A number of centrally acting sympathomimetics such as phentermine, cathine and diethylpropion continue in short‐term use. A serious realization throughout the majority of these approaches is the typical lack of ability to accomplish placebo-adjusted mean weight reduction above 10% of initial body weight when persistantly administered at bearable dosages. As better weight management is accomplished, it is normally gone along with by different significant severe or persistent negative effects34 (Table 1). Table 4 compares stage III trialdata for currently offered medications consisting of percent weight loss, percent ofintent to deal with (ITT), completers that lost 5% and 10% of body weight, andpercent of topics that quit of study. The course followed in the development of gut-hormone acquired agents for excessive weight therapy has parallels in the development of other anti-obesity drugs. Tesofensine is a triple natural chemical re-uptake inhibitor that acts on the central nerves to boost efficacy contrasted to solitary re-uptake preventions such as bupropion and rimonabant. Likewise, the mix of three Sirt1 and AMPK agonists (Sildenafil, leucine, and metformin) uses a tiny dose of metformin to boost the weight lowering impact of metformin alone while lessening the gastrointestinal results it typically induces. At this dose, metformin does not create sufficient weight reduction to get authorization as a stand alone treatment. However, the key objective is to supply a point of view on the state of the science as it relates to the pipeline of emerging treatments for weight problems.Social Links