
September 5, 2024
Obesity Medications In Development Pmc
What Is The Pipeline For Future Medicines For Excessive Weight? It additionally impaired their ability to be turned on by an open loop optogenetic stimulation (Fig 3). Making use of lean Vgat-ChR2 mice, we found that tesofensine reduces the feeding actions induced by the optogenetic activation of LH GABAergic nerve cells (Fig 4). Furthermore, in Vgat-IRES-cre obese computer mice, just a greater tesofensine dosage could subdue optogenetically caused feeding, suggesting that, throughout weight problems, LH GABAergic nerve cells seem to be hypersensitized. Alternatively, the chemogenetic inhibition of LH GABAergic nerve cells potentiates the anorexigenic results of tesofensine (Fig 6).- Sibutramine, a norepinephrine and serotonin reuptake prevention that actsby decreasing food intake, was approved in 1997 for the lasting treatment ofobesity.
- In rats, CRMP was utilized to achieve low-level hepatic mitochondrial uncoupling that turned around hypertriglyceridemia, insulin resistance, hepatic steatosis and diabetes264.
- No matter treatment with metreleptin, T cell lymphoma has been reported in individuals with gotten generalised lipodystrophy.
- Novel or combined approaches to manage hypothalamic excessive weight are hence needed to attain reliable and sustained weight management.
Discovering The Possibility Of Rapamycin In The Therapy Of Psoriasis
This power intake reduction after mixed hormonal agent administration was a lot more noticable than during infusions of either hormonal agent alone. The efficacy and security of cetilistat, an unique prevention of intestinal lipases, was established in both obese nondiabetic (24) and diabetic person (25) clients. Weight reductions (from − 3.3 kg to-- 4.3 kg) accomplished by the treatment with different dosages of cetilistat (60 mg t.i.d., 120 mg t.i.d., 240 mg t.i.d.) over a 12-week period were statistically substantial compared to sugar pill (24,25). The therapy with cetilistat resulted in substantial reductions in total and LDL cholesterol degrees in obese clients (24) and in an enhanced glycemic control in obese clients with diabetes (25 ).What is the brand-new medication to combat fat?
Wegovy is the brand name for a medicine called semaglutide. It is approved for use in the NHS, along with diet and exercise, to take care of excess weight and excessive weight in some individuals. It is only readily available via expert weight monitoring clinics.
4 The Role Of Insulin And Leptin In The Control Of Feeding, And Power Homeostasis
At the core of tesofensine's device of activity lies its capability to exactly target and regulate specific natural chemicals in the brain. By hindering the reuptake of norepinephrine, dopamine, and serotonin, tesofensine elevates the degrees of these critical neurotransmitters, exerting an impressive impact on cravings control, power expenditure, and fat storage space. On the other hand, just the greater dosage of 6 mg/kg caused strong tongue activities in the air, and this stereotypy showed some resemblances with phentermine.Comparison Of Tesofensine With Various Other Appetite Suppressants
Heart disease, cancer, and stroke are the leading reasons of death worldwide, in recent times [1] These conditions relate to the "epidemic of weight problems," one of the significant global health and wellness problems [2] In particular, lockdown measures to limit the transmission of coronavirus have adversely influenced a range of weight management techniques, including physical activity and healthy eating. Thirty 2 healthy males were treated with 2mg/d of tesofensine for1 week and after that randomized to l. 0mg/d or placebo for one more 7 days. Even whileattempting to maintain food consumption, subjects shed 1.8 kg over the 2 weeks.Tesofensine therapy enhanced aesthetic analog scale scores of satiety andincreased 24 hr Helpful hints fat oxidation about placebo. Although an adjustment in totalenergy expense was not found, resting power expenditure wassignificantly higher. 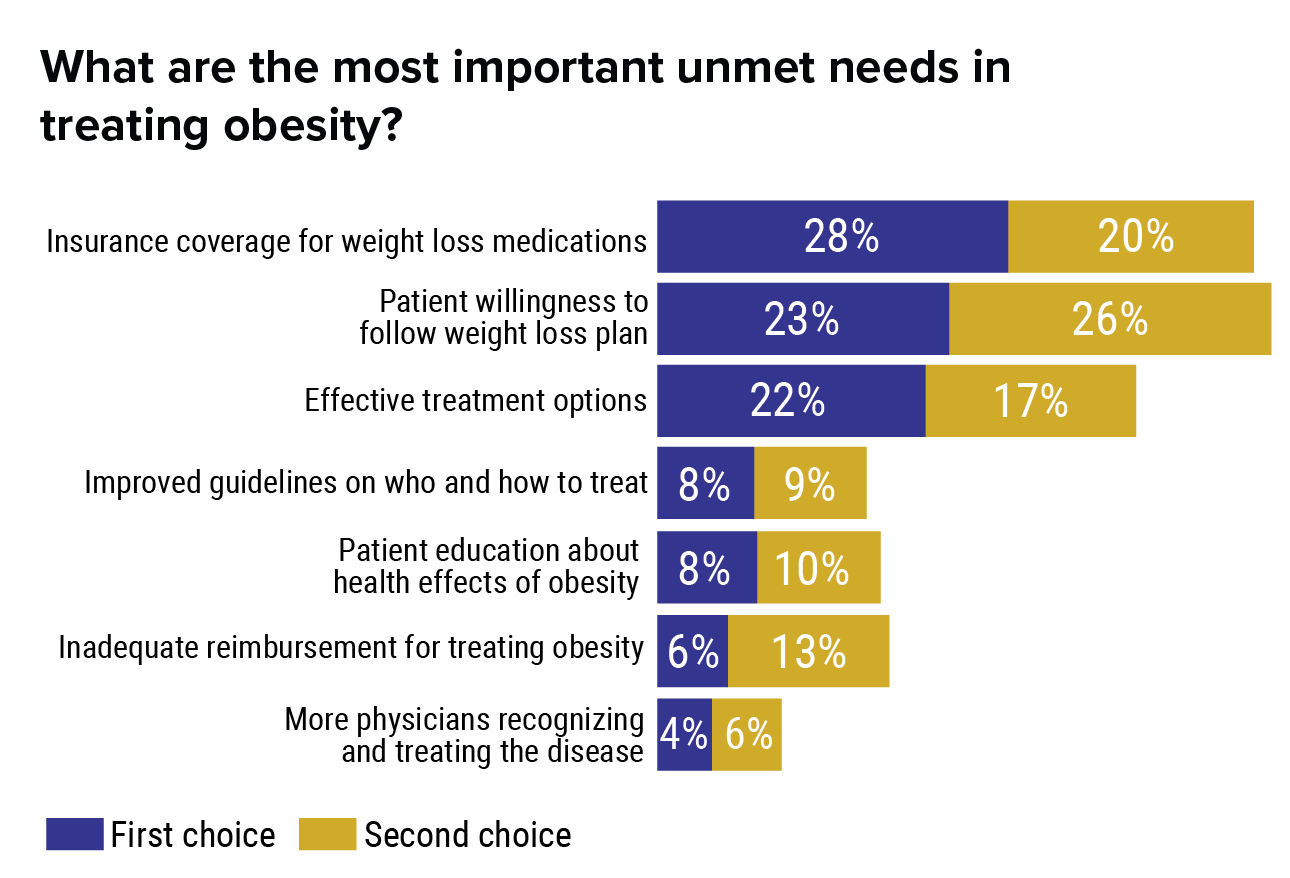

Social Links