September 5, 2024
Having A Hard Time To Accomplish Fat Burning Goals? Uncover The Power Of Tesofensine And Glp-1 Agonists!
Pharmaceuticals Free Full-text Pharmacological Treatments And Natural Biocompounds In Weight Administration Excessive weight wasnot recognized as a chronic condition up until 1985 by the scientific neighborhood and2013 by the medical community. Pharmacotherapy for obesity has advancedremarkably considering that the first class of drugs, amphetamines, were approved forshort-term use. A lot of amphetamines were removed from the obesity market due toadverse occasions and potential for dependency, and it emerged that obesitypharmacotherapies were required that might safely be provided over thelong-term. This testimonial of central nervous system (CNS) acting anti-obesity drugsevaluates current therapies such as phentermine/topiramate which act throughmultiple natural chemical paths to reduce cravings.Is Tesofensine Fda Accepted?
Does tesofensine cause clinical depression?
weight-loss, and 32%of obese individuals had & #x 2265; 5%weight loss adhering to 14 wk of treatment. Weight reduction was come with by hypophagia, recommending a hunger suppressant activity. Prevent Unfavorable Medicine Occasions Today Tesofensine is a Serotonin-norepinephrine-dopamine-reuptake-inhibitor(SNDRI). SNDRIs are a class
of psychedelic antidepressants. Although shedding 10 kg in 1 month is a huge challenge and fairly tough, you can still do it.

- Losing also a small amount of weight can have significant benefits, consisting of better blood pressure, blood cholesterol, and blood sugar level levels.
- It can be speculated that as raised high blood pressure was predictable from its setting of activity, this may have been taken care of with reduced doses and a much more adaptable application routine.
- Aminorex was eliminated from the marketin 1968 due to its organization with primary pulmonary hypertension and by 1972the occurrence of main pulmonary high blood pressure had been up to the level priorto the release of aminorex [11]
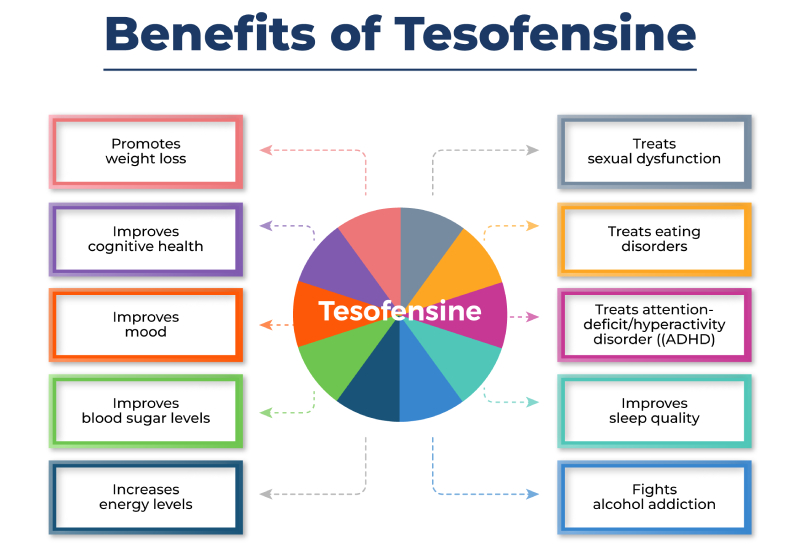
- A three-way monoamine reuptake prevention, tesofensine (NeuroSearch), has created appealing lead to stage II clinical tests.
Tesofensine
A current sophisticated pharmacological examination disclosed the one-of-a-kind profile for tirzepatide as an unbalanced agonist due to greater fondness and strength at the GIP receptor (GIP-R) versus GLP-1R along with a biased agonist at the GLP-1R while maintaining full agonism at the GIP-R [59] The level of HbA1c reduction and weight decrease observed in pre-clinical, stage 1 and 2 professional trials has not previously been observed in diabetes mellitus clinical trials. 3 various 8-week dose-escalation programs adhered to by 4-week dosing of 12 or 15 mg have actually been checked in order to choose therapeutic doses and dose-escalation steps for examination within the phase 3 researches of tirzepatide [61] The stage 3 SURPASS medical trial programme consisting of ten researches is testing the theory that tirzepatide therapy gives comparable effectiveness, safety and cardio results in the management of type 2 diabetes [62]Tesofensine Peptide Evaluation: Benefits, Results, Dosage, & A Lot More
However, the main issues for qnexa such as cognitive disorder, psychiatric events and teratogenicity originate from the topiramate content. The recent FDA review focused on these issues and requested better evidence of safety and security going beyond the 1 year duration researches that had actually been carried out to day. Supplying such information for either qnexa or any type of future entries is most https://Clinical-trials.b-cdn.net/Clinical-trials/product-quality/how-tesofensine-urges.html likely to show a substantial financial obstacle with no assurance of an effective outcome. Amylin produced by pancreatic β-cells acts to decrease post-prandial glucagon secretion, slow gastric draining, and centrally boost satiety [88] Early research studies revealed that pramlintide usage in patients with insulin-treated diabetes improved glycemic control and sustained weight decrease by reducing food consumption [89] Tesofensine was originally developed for the therapy of Alzheimer's and Parkinson's condition. It showed restricted performance for those applications but revealed potential for weight loss therapy. In a stage II clinical trial, obese patients received 0.25, 0.5, or 1 mg of tesofensine or sugar pill over 24 weeks after a 2 week run-in duration (Astrup et al., 2008). Outcomes of this trial revealed substantial weight reduction in all doses when compared to placebo.Social Links