
September 5, 2024
Tesofensine Expertise And Referrals
Tesofensine, A Novel Antiobesity Medication, Silences Gabaergic Hypothalamic Nerve Cells Plos One The outer sympathomimetic activity of sibutramine brings about an increase in both systolic (sBP) and diastolic high blood pressure (dBP) and pulse rate. However consolidated evaluation of two placebo-controlled tests wrapped up that sibutramine treatment is not likely to elicit a vital increase in high blood pressure also in hypertensive individuals with well-controlled hypertension. This is clarified by the clonidine-like effect of sibutramine, which is moderated through activation of main α-2 adrenoreceptors (12 ). It needs to be pointed out that the reduction of high blood pressure in individuals with type 2 diabetes after orlistat treatment was less obvious and the boost in blood pressure after sibutramine was higher.- If an anticipating correlate in between metabolic profiling and propensity to weight management can be developed, this can have a profound impact on the future of healthcare in excessive weight.
- GIP regulation of basal metabolism continues to be enigmatic as activation and stopping of the GIPR receptor have both been shown to decrease body weight48.
- Postprandial GLP-1 secretion is lowered in diabetic person individuals compared with nondiabetic individuals.
- With a deep understanding of integrative functional medicine and the complexities of weight problems, our professionals go to the forefront of the field.
Medical Weight-loss
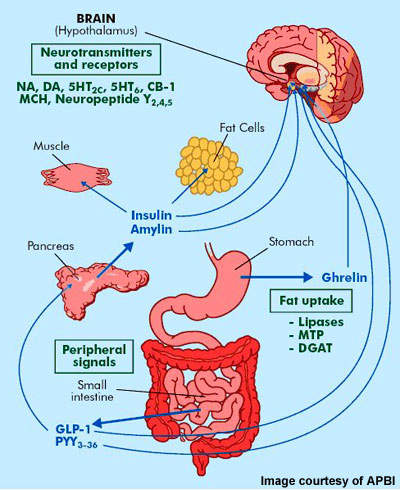
This energy consumption reduction after consolidated hormonal agent management was much more obvious than during infusions of either hormone alone. The efficacy and safety of cetilistat, an unique prevention of stomach lipases, was established in both obese nondiabetic (24) and diabetic person (25) people. Weight reductions (from − 3.3 kg to-- 4.3 kg) accomplished by the treatment with various dosages of cetilistat (60 mg t.i.d., 120 mg t.i.d., 240 mg t.i.d.) over a 12-week period were statistically considerable compared to sugar pill (24,25). The therapy with cetilistat led to significant reductions in overall and LDL cholesterol levels in obese clients (24) and in a boosted glycemic control in overweight individuals with diabetes (25 ).What are the sophisticated weight problems medications?
Zepbound (tirzepatide), Wegovy (semaglutide), Saxenda (liraglutide), and more are currently FDA authorized as weight-loss treatments.
Evommune Enrols First Subject In Chronic Inducible Urticaria Treatment Test
Tesofensinetreatment stabilized the dopamine degrees in the DIO rats, but had no result onthe chow-fed pets, suggesting that the anti-obesity results of tesofensineare due, a minimum of in part, to positive modulation of main dopaminergicactivity [119] Because the major negative occasions bring about discontinuation in theproof-of-concept trial were nausea and throwing up attributable to naltrexone, a24-week stage II test examined 3 doses of naltrexone with bupropion tofind the most bearable dose with adequate efficacy. The trial randomized 419obese based on bupropion alone 400 mg/d, 3 mix doses ofnaltrexone/bupropion (NB) with naltrexone at 16 mg/d, 32 mg/d, or 48 mg andbupropion 400 mg/d, or placebo [38] Theplacebo subtracted weight management was greatest (4.65% of body weight) in the NB 32mg/d team by last monitoring continued (LOCF) evaluation due to higherdrop outs in the NB 48 mg/d team from nausea and throwing up [38] In a sub-study of this trial, overall and visceralfat was gauged by dual energy x-ray absorptiometry (DXA) in a part of 107participants. In the eighty topics that completed the sub-study, there was agreater reduction in complete body fat (NB 14% vs. sugar pill 4%) and natural fat (NB15% vs. 4.6%) in the NB mix group compared to sugar pill or bupropion alone [39]Comparison Of Tesofensine With Other Cravings Suppressants
Nonetheless, a current meta-analysis revealed that amongst all the FDA-approved anti-obesity drugs, liraglutide had the greatest (13% of study individuals) price of discontinuation as a result of its adverse effects complied with by naltrexone/bupropion (12% of research study participants) [51] At first, there were concerns about the risk of severe pancreatitis; nevertheless, lasting tests reported that the danger does not significantly increase with making use of liraglutide [52, 53] Although the biomarkers, such as amylase and lipase, of severe pancreatitis rose in a non-dose-dependent manner throughout the therapy with GLP-1 receptor analogs, their rise was not gone along with by signs and acute pancreatitis was not identified when kept an eye on even more [54] However, researches on rats revealed the proliferative effect of liraglutide on thyroid C-cells; hence, contraindications for liraglutide include individuals with (or with a family background of) medullary thyroid carcinoma or type 2 numerous endocrine neoplasia [29] A stage 3b RCT revealed no distinction in the calcitonin degrees and price of medullary thyroid carcinoma between the placebo- and liraglutide-treated (≤ 1.8 mg) groups, during a follow-up after 3.5 years [55] Pancreatic, intestinal tract, and breast tumors were much more regularly established in rats administered with incretin-based medications; however, these results were not verified in human researches [56,57,58] In a rat design of diet-induced weight problems (DIO), tesofensine treatmentproduced robust weight-loss come with by hypophagia. To recognize the neuralpathways modulating weight management and hypophagia, turnaround of View website these effects wasinvestigated utilizing various monoaminergic receptor antagonists co-administeredwith tesofensine. Tesofensine substantially lowered food consumption in the first 12hours of administration in a dose dependent manner, with a maximum impact after3 days. The hypophagic effect gradually dissipated and returned to regulate levelsby day 15, but the decrease in body weight continued throughout of the 16day experiment. 
Social Links