
September 5, 2024
Comprehensive Evaluation Of Current And Upcoming Anti-obesity Medications Pmc
Pharmaceuticals Totally Free Full-text Existing Treatments In Professional Tests Of Parkinsons Illness: A 2021 Upgrade A caveat to this last searching for is that the reduction of YFAS ratings within 24 h might be quicker than pexacerfont's anticipated time course of CNS activity. Generally, the outcomes offer rationale for well-powered tests of CRF1 receptor antagonists to reduce uncontrollable eating (Epstein et al., 2016; Spierling and Zorrilla, 2017). Given that tesofensine is a three-way reuptake inhibitor that manages the level of DA, 5-HT, and NE throughout the entire mind, its results are anticipated to be dispersed and brain-wide, definitely not restricted to LH or GABAergic neurons. Refresher courses utilizing high-density recordings of neuropixels need to reveal just how distributed tesofensine's effects are throughout the brain. Hereof, the equilibrium of neurotransmitters in the brain, especially norepinephrine (NE), dopamine (DA), and serotonin (5-HT), is a significant determinant of the total weight loss properties of the majority of cravings suppressants [14, 25, 64] A caveat of our research study is that we did not measure the launch of these neurotransmitters.- The very first stimulant to be supported by the FDA for the treatment of excessive weight was methamphetamine in 1947 (United States Fda, 2012).
- At 24 weeks, people had actually revealed no evidence of plateau, which suggested that better weight loss could be attained in a year-long test.
- Furthermore, making use of tesofensine triggers desirable changes in waistline area, insulin resistance, adiponectin, lipid profiles, and glycemic control.
- Data in panel a refer to liraglutide 3 mg (ref.176), orlistat289, naltrexone/bupropion292, phentermine/topiramate291, semaglutide 1 mg (ref.125), semaglutide 2.4 mg (ref.38) and tirzepatide (5 and 15 mg) 126.
- As a matter of fact, sibutramine produced no discernable subjective impacts at low dose and was dysphoric and aversive at high dose (Cole et al., 1998; Schuh et al., 2000).
- Among the likely relevant underlying mechanisms is a decline in peripheral adiposity signals (leptin, insulin) following fat burning, and extended fasting leads to boosted expression and sensitization to orexigenic neuropeptides in the hypothalamus and the hindbrain.
Is tesofensine accepted by the FDA?
The FDA granted orphan medication classification for fixed-dose mix of tesofensine and metoprolol in PWS in March 2021 and hypothalamic obesity in July 2021. Tesofensine is a centrally acting monoamine reuptake inhibitor that obstructs the presynaptic reuptake of dopamine, serotonin, and noradrenaline.
Experts Discuss Research Right Into A Feasible New Obesity Medicine, As Released In The Lancet
Sibutramine is an interesting molecule due to the fact that it consists of the β-phenylethylamine underpinning that is present in several monoamine launching agents, eg d-amphetamine, methamphetamine and MDMA. Furthermore, sibutramine's energetic metabolites Click here for more info prevented the reuptake of noradrenaline (norepinephrine), 5-hydroxytryptamine (5-HT, serotonin) and dopamine (Cheetham et al., 1993, 1996; Heal et al., 1998b), which elevated the inquiry of its medicinal resemblance to drug. Therefore, a huge amount of preclinical and scientific screening was done to attempt to demonstrate that sibutramine was pharmacologically different from both d-amphetamine and cocaine.A Worldwide Annual Study Of New Information In Negative Medicine Responses
The weight problems crisis has gotten to the stage that strong factor to consider must be offered to ample application of this effective and inexpensive class of medicine. Scientific tests reviewing the effectiveness of Tesofensine for weight reduction have actually generated encouraging outcomes. In one research study entailing obese individuals, Tesofensine therapy resulted in a mean weight-loss of about 10% of preliminary body weight after 24 weeks of therapy. In the 1950s and 1960s dexamphetamine was widely suggested for a variety of problems including weight problems, clinical depression, and poor inspiration (Kiloh and Brandon, 1962). Although it was acknowledged that it may periodically be taken as a behavior to recover confidence it was generally thought about secure also for long-lasting usage (Content, BMJ, 1955). Nonetheless, it emerged that some individuals were abusing dexamphetamine and had actually been fraudulently getting numerous prescriptions and having them given by different drug stores (Kiloh and Brandon, 1962). A couple of were admitted to healthcare facility with psychosis and lack of nutrition, experiencing clinical depression on medicine withdrawal. Then the viewpoint suddenly turned versus the energizers for the treatment of obesity (USA Fda, 2012). In spite of this, the energizer phentermine has actually continued to be certified for short-term usage in weight problems and in mix with the anticonvulsant topiramate for long-term usage. Additionally, when people with obesity that try to slim down suddenly lower their food consumption, some experience severe bowel irregularity owing to reduced dietary fiber intake. Constipation can be treated by orlistat, in addition to dietary fiber supplementation, by means of its stomach adverse effects. The weight management moderated by lorcaserin is likewise similar to present therapy and its tolerability appears plain with 40-- 45% of people discontinuing treatment over 52 weeks. 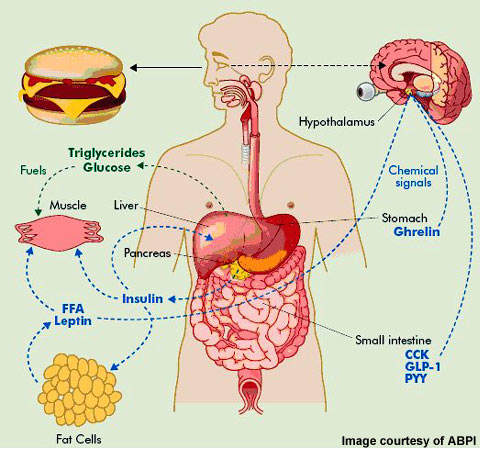
Social Links