
September 5, 2024
Pharmaceuticals Free Full-text Medicinal Therapies And All-natural Biocompounds In Weight Monitoring
B Boosts Cognitive Health
What is the negative effects quick fat burning?

- The major adjustment observed during the tesofensine treatment was a shift in the circulation of tests completed on each quartile.
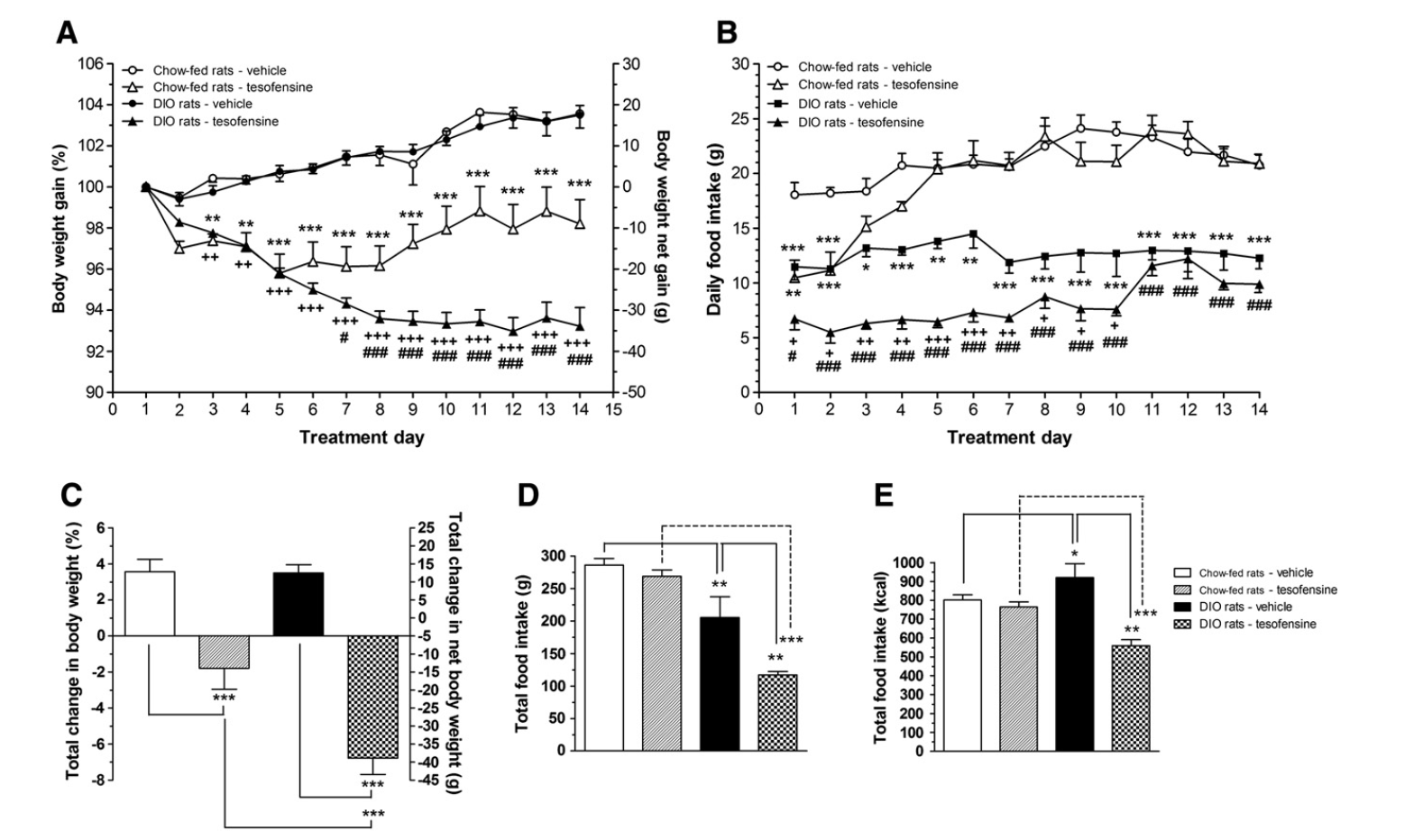
- The highest dose of PRX carried out (10 mg/kg, ip, quote) produced a substantial reduction of food consumption in the animals for practically all of the 6 week treatment period.
- A video was tape-recorded at 60 structures per 2nd (fps) with a resolution of 1280 x 720 pixels utilizing a Kayeton video camera (version KYT-U400-MCS2812R01).
- In a double-blind, placebo-controlled research released in the journal Obesity, researchers located that individuals taking tesofensine lost substantially extra bodyweight than those that got a placebo.
- Nevertheless, tesofensine seems to enhance the recruitment of LH neurons exhibiting activation after medication management (i.e., see E4 neurons in Fig 2).
What Is Tesofensine?
Electrophysiological recordings further uncovered that NPE evoked a solid inflection on NAcSh's single-unit and populace task that associated with the beginning of the active awake mind state, a sign of sleep problems. Given that the significant adverse events causing discontinuation in theproof-of-concept test were queasiness and vomiting attributable to naltrexone, a24-week stage II test evaluated three dosages of naltrexone with bupropion tofind the most bearable dosage with enough efficiency. The trial randomized 419obese based on bupropion alone 400 mg/d, 3 mix doses ofnaltrexone/bupropion (NB) with naltrexone at 16 mg/d, 32 mg/d, or 48 mg andbupropion 400 mg/d, or placebo [38] Theplacebo subtracted weight-loss was best (4.65% of body weight) in the NB 32mg/d group by last observation carried forward (LOCF) analysis due to higherdrop outs in the NB 48 mg/d team from nausea or vomiting and vomiting [38]Tesofensine Peptide Testimonial: Benefits, Results,
However, the primary problems for qnexa such as cognitive dysfunction, psychological occasions and teratogenicity originate from the topiramate material. The current FDA evaluation focused on these concerns and asked for even more evidence of security surpassing the 1 year duration studies that had actually been performed to date. Providing such information for either qnexa or any type of future entries is most likely to prove a significant financial obstacle without guarantee of an effective end result. Amylin secreted by pancreatic β-cells acts to minimize post-prandial glucagon secretion, slow-moving gastric emptying, and centrally enhance satiety [88] Very early studies showed that pramlintide use in clients with insulin-treated diabetes mellitus boosted glycemic control and supported weight reduction by lowering food consumption [89] The mind was cut, and sections of 40 μm were installed in Dako fluorescence mounting tool. Nevertheless, the neuropeptide approach appears to hold considerable assurance and numerous neuropeptide ligands that are presently in scientific advancement are taken into consideration listed below. Right Here at Heritage Health and Wellness Center with places in Cedar Hill and Dallas, Texas, our board-certified physician and the owner Ifeoma Ogbonna, MD, gives customized weight https://nyc3.digitaloceanspaces.com/pharmaceutical/pharmacy-benefit/product-sustainability/extensive-evaluation-of-existing-and-forthcoming-anti-obesity-medicines.html administration programs to assist those who require or want to drop weight.Social Links