September 5, 2024
Tesofensine, A Novel Antiobesity Medication, Silences Gabaergic Hypothalamic Nerve Cells
Medical Care Free Full-text Medicinal Support For The Therapy Of Weight Problems Present And Future At 24 weeks, clients had shown no evidence of plateau, which suggested that better fat burning might be accomplished in a year-long trial. This research found that tesofensine induced greater weight reduction in obese rats than in lean Wistar rats. We assumed that this was as a result of tesofensine's capacity to regulate neuronal task in the LH.Diversification Of Individual Associates
Is tesofensine an antidepressant?
- For CNS medicines being checked in obesity tests, brand-new approaches of determining suicidality and other psychiatric dangers may offer not only a lot more exact safety information, but also a much better chance at approval.
- Negative occasions were consistent with the pharmacodynamic account of Tesomet, consisting of frequent event of dry mouth, rest disturbances, lightheadedness, and migraine.
- However, this research was halted by the NIH IRB as a result of factors unconnected to adverse medication effects or effectiveness (reinterpretation of the Common Policy for human subject defense under HHS, 45 CFR 46A).
- Style A pilot phase 2, randomized, double-blind, placebo-controlled, parallel-group trial.
- Isobolographic evaluation was implemented to establish if the communication in between two medicines given in combination is collaborating (supra-additive), additive, or antagonistic (infra-additive) [26, 27]
Comprehensive Review Of Current And Future Anti-obesity Medications
Furthermore, naltrexone ER/bupropion ER is contraindicated in patients with a history of convulsive seizure or bipolar illness. For patients with psychological or mental disorders that take antipsychotics or antidepressants, caution is required owing to the capacity for medicine communications and raised risk of seizures [33] A range of (three-way) reuptake inhibitors of NE, DA and 5-HT have been investigated for the treatment of weight problems, depression and ADHD (Discovered et al., 2012; Schoedel et al., 2010). These medicines are not distinctly triple uptake inhibitors since the majority of stimulants have activity at these uptake procedures. Two abuse possible studies have actually been reported for this course of substances-- one with tesofensine (Schoedel et Look at this website al., 2010) and the other with GSK (Found out et al., 2010).Global Individuals
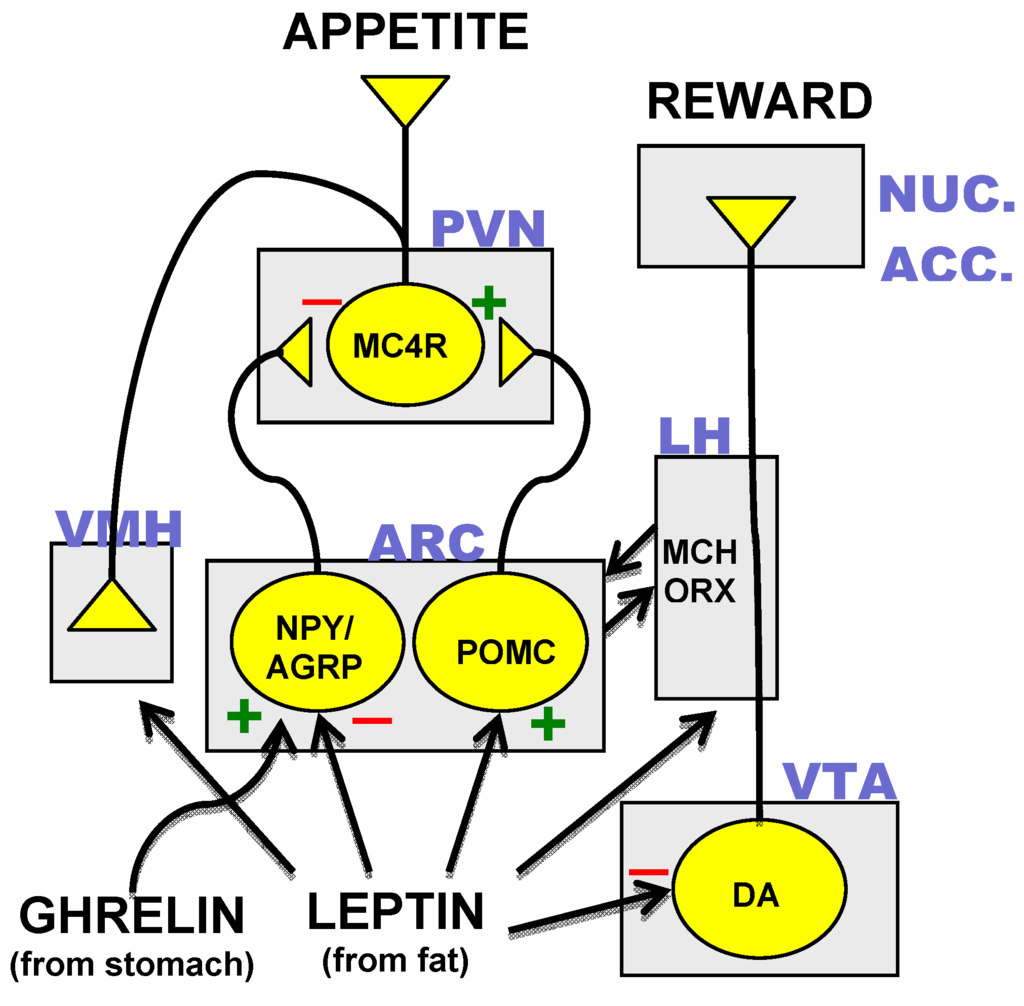
Among the likely appropriate underlying mechanisms is a decline in peripheral adiposity signals (leptin, insulin) following weight reduction, and extended fasting causes raised expression and sensitization to orexigenic neuropeptides in the hypothalamus and the hindbrain. Simultaneously, the expression of and level of sensitivity to anorexigenic neuropeptides decrease in these same locations to make up a double-barrelled support of body weight111,112,113. Concurrently, the density and stamina of the orexigenic agouti-related peptide (AgRP)/ neuropeptide Y (NPY) fibers that forecast from the arcuate nucleus (ARC) to the paraventricular hypothalamic centers boost in action to extended fasting. This remodelling of the ARCAgRP/NPY forecasts associates with increased activation of paraventricular hypothalamic nuclei neurons with the objective to restore food intake114. One more barrier in weight loss pharmacology is that consistent altitude of adiposity signals such as leptin and insulin lead to desensitization, bring about a damaged responsiveness of this homeostatic system115,116,117. A striking searching for sustaining this point of view is that leptin supplementation reveals exceptional effectiveness in lowering body weight in people with hereditary leptin deficiency96,118,119, yet is greatly ineffective in more typical polygenetic types of obesity115,116,117. As a boost in blood pressure is observed at high dosages, it is important to demonstrate the security of tesofensine in a massive scientific test. The most efficacious currently available treatment for excessive weight, sibutramine, has the ability to generate an average body weight-loss of 4.45 kg over a 52 week duration (Li et al., 2005) however is no more available in Europe. Of the different treatments in late stage clinical tests, qnexa and tesofensine, show up to supply the most considerable renovations in efficiency over sibutramine (Table 3). Of these, qnexa appears to be one of the most effective, with the highest possible dose achieving approximately 10 kg (9%) placebo-adjusted fat burning over 52 weeks with over 60% of individuals shedding over 10% of their weight adhering to an LOCF analysis. 

Social Links