
September 5, 2024
Tesofensine, A Novel Antiobesity Drug, Silences Gabaergic Hypothalamic Neurons

Medicines En Route To Take On Obesity Epidemic Orexigen expects to file an NDA in the initial half of 2010, according to a company news release. Of key interest is why GLP1R agonism works so well and how GIP might synergize with GLP1 to improve fat burning. Short of the outcomes that have been accomplished in vivo, most significantly the 6-month and 1-year clinical studies that appear to suggest significant additional benefits of semaglutide when compared to liraglutide, it is hard to ascribe a molecular basis for that difference. These two agents are both highly potent and careful GLP1R agonists, likewise fatty acylated, that give continual drug plasma concentrations when utilized as prescribed. The difference is not merely a matter of extensive time action as also a long-action Fc agonist such as dulaglutide does not match the body weight decreasing of semaglutide284. The cost-effectiveness of such treatment would be very depending on the cost of the medication. The price of developing a brand-new medicine has been approximated at $2.6 billion (Avorn, 2015), which has to be recuperated prior to the license expires. Tirzepatide is part of a brand-new course of drugs called called GLP-1 receptor agonists that were created to deal with type 2 diabetes. Furthermore, fMRI information suggest that naltrexone/bupropion treatment might boost the control of eating habits [66] Little clinical information are readily available on the results of phentermine/topiramate ER on consuming behavior. The negative effects experienced by more than 20% of participants who utilize orlistat for 2 years include fecal incontinence, oily identifying, and fatty stool. In one study, the treatment discontinuation price was 8.8% in the treatment team and 5.0% in the placebo team [19,20]
- Three individuals experienced significant negative events (SAEs); 2 randomized to Tesomet and one to sugar pill.
- Lastly, we discovered that the appetite suppressant effect of tesofensine is not due to the induction of preference aversion.
- We satisfaction ourselves on our ingenious and tailored method to weight reduction, and our application of advanced therapies like Tesofensine and semaglutide therapy exhibits our dedication to supplying extraordinary outcomes.
- It is feasible that the administration of a particular mix of these medications will certainly have beneficial results on the facility physiological factors that contribute to the upkeep body weight.
Weight Problems
Initially created as a treatment for Alzheimer's disease and Parkinson's illness, its capability to subdue appetite and induce weight loss was serendipitously uncovered throughout clinical tests. To conclude, theADVANS research study offered some indicators of an antiparkinsonian task of the dopamine reuptake inhibitor tesofensine in innovative PD. The reliable dosages of 0.25 mg/d and 0.5 mg/d exhibited an acceptable security account, while greater does might induce unfavorable reactions of professional problem in this older population. These pilot results deserve additionally expedition to far better analyze the benefit-risk proportion of tesofensine in the treatment of PD.Data Accessibility
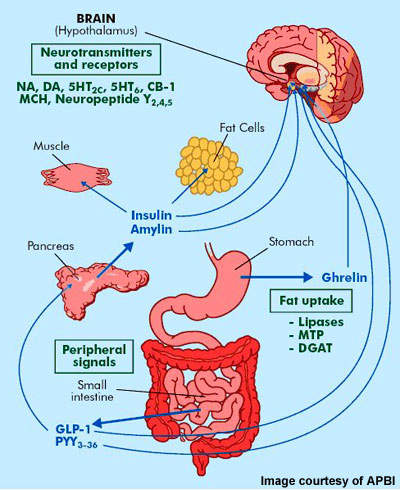
Amylin turns on specific receptors including those of the calcitonin gene-related peptide (CGRP). Although the significant effect of amylin on basal metabolism is moderated with enhancing satiation, amylin has actually likewise been shown to affect hedonic control of consuming, including a decrease in feeding incentive neurocircuits233. Nevertheless, the medical application of native amylin in dealing with weight problems has been shadowed by physical accumulations connected to pancreatic island death in humans234, a finding not observed with rat amylin235. The anorexigenic potential of amylin promoted the development of pramlintide, a rat-based synthetic analogue of amylin236. A more comprehensive metabolic and genetic characterization in mix with detailed illness aetiology and feedback to various systems in drug action must bring about a renovation in person treatment. Furthermore, this can likewise possibly cultivate the next generation of AOMs by progressing a much deeper understanding into the molecular pharmacology of body weight guideline. FDA called once more on the supervisor of the Center for Suicide Risk Assessment when it saw prospective dangers of self-destructive ideation with rimonabant. Posner's team took into consideration an overall of 1,201 "client narratives" from seven rimonabant trials. Utilizing C-CASA, they identified 91 cases as either "potentially" or "most definitely" self-destructive, however got rid of some because they took place in research study arms without placebo control. The final tally of suicidality instances was 74, with 20 on placebo, 8 on rimonabant 5 mg, and 46 on rimonabant 20 mg; the overall drug-to-placebo proportion was 1.8 to 1.What are the threats of taking tesofensine?
Tesofensine 0.25 mg, 0.5 mg, and 1.0 mg and diet plan caused a mean weight loss of 4.5% (0.87 ), 9.2% (0.91 ), and 10.6% (0.84 ), specifically, higher than diet plan and placebo (p<
Social Links