September 5, 2024
Tesofensine, An Unique Antiobesity Medication, Silences Gabaergic Hypothalamic Nerve Cells Pmc
Tesofensine, An Unique Antiobesity Medicine, Silences Gabaergic Hypothalamic Neurons The research recommend the experimental drug is safe due to the fact that it had no effect on high blood pressure and only elevated heart rate somewhat, claimed Arne Astrup of the University of Copenhagen, that led the research released in the journal Lancet. Individuals taking NeuroSearch A/S's weight problems pill tesofensine lost two times as much weight as males and females making use of authorized weight-loss medicines, Danish scientists said on Thursday. Tesomet resulted in mathematical enhancements in the physical part scores of SF-36 from week 4 to week 20; nevertheless, returned to near standard at week 24, while the sugar pill team after an initially reduced physical part rating at week 8, went back to near baseline at week 16 (Fig. 7A). Both groups had numerical reductions in mental part rating to better level in Tesomet-treated individuals (Fig. 7B). In total, 35 people were screened, of whom 21 one-of-a-kind clients (16 ladies) satisfied qualification criteria and were randomized (Fig. 2).A Globally Yearly Survey Of Brand-new Information In Damaging Medication Responses
For how long does tesofensine stay in your system?
- Lastly, one Tesomet-treated client had re-growth of craniopharyngioma discovered by a pre-scheduled MRI-scan.
- Of these, qnexa seems the most effective, with the highest dosage achieving approximately 10 kg (9%) placebo-adjusted weight-loss over 52 weeks with over 60% of participants losing over 10% of their weight complying with an LOCF evaluation.
- The Mayo group carried out a yearlong professional test done in a weight administration facility where 312 individuals were arbitrarily assigned to phenotype-guided therapy or treatment that was not phenotype led and consisted of anti-obesity drugs.
Safety
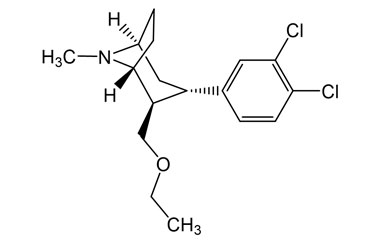
The three-way mechanism of activity, however, might present severe side-effect issues in massive tests. The medicine was introduced in 18 EU nations, beginning with the UK in June 2006, under EMEA's conditional authorization. However records of psychiatric adverse effects restricted its usage, omitting clients with major depression. According to Wolters Kluwer, in May 2008, as adverse-events records accumulated, the European firm upgraded the label to indicate that clinical depression may take place as a negative effects in patients without symptoms other than excessive weight. After FDA released an approvable letter in February 2006, the agency's advisory board elected 14-0 against suggesting authorization just 4 months later, specifying that Sanofi had fallen short to supply adequate safety information to show that rimonabant's advantages surpassed its risks. " The potential market for this medication and the continued unpredictability about its threats, both recognized and unknown, lead to our problem concerning the use of this drug in the basic populace," FDA team clinical customer Amy Egan told The New York Times. Craniopharyngioma, one of the most typical source of hypothalamic obesity, has a total incidence of approximately 1.3-- 1.7 per million people/year (8, 9). Hypothalamic excessive weight establishes in roughly 50% of craniopharyngioma survivors (10, 11). The major negative effects of liraglutide are intestinal signs, such as queasiness, diarrhea, constipation, and throwing up, and it is advised that the dose is incrementally enhanced to decrease the occurrence of these unfavorable occasions. Owing to the delayed gastric emptying triggered by liraglutide, the action of other medicines can be impacted. Furthermore, liraglutide use can create gallstones and, less frequently, acute pancreatitis [57,58]; it ought to not be made use of in clients with a background of pancreatitis. Since there are worries relating to liraglutide usage and medullary thyroid cancer cells and numerous endocrine neoplasia, it ought to not be made use of in clients with a previous or family background of such conditions [59-- 61] Persistantly elevated blood glucose as a result of inadequate action or manufacturing of insulin. Tesofensine jobs by interfering with three brain chemicals-- noradrenline, serotonin and dopamine-- associated with managing cravings. "We need to for that reason be a little scrupulous about accepting these cases regarding efficiency and await the results of the more pertinent https://seoneodev.blob.core.windows.net/pharma-warehousing/compounding-pharmacy/product-strategy/pharmaceuticals-free-full-text-current-treatments-in-medical-trials-of.html Phase III studies, which the writer does say at the end of the paper," Ian Mop, a researcher at Robert Gordon University in Britain said in a declaration. The World Wellness Company categorizes around 400 million people around the world as overweight, representing a significantly profitable market for medicine manufacturers. 
Social Links