
August 27, 2024
Esophagogastric Anastomosis In Rats: Boosted Healing By Bpc 157 And L-arginine, Aggravated By L-name
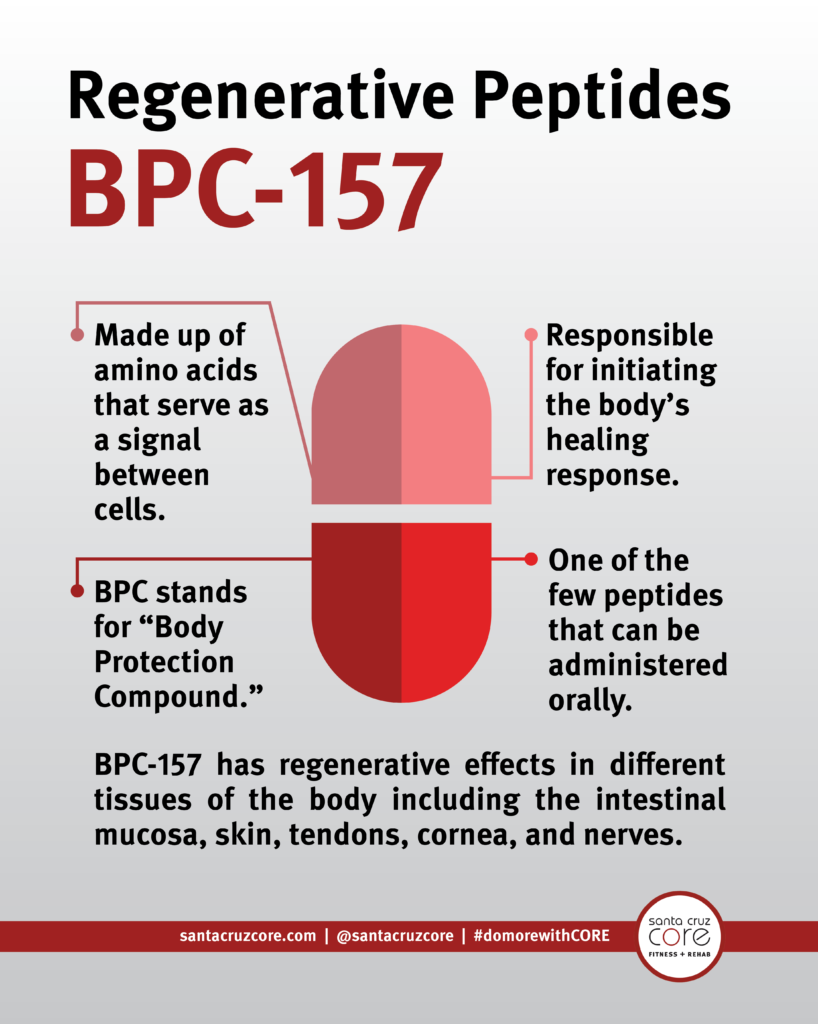
Gastric Pentadecapeptide Bpc 157 As An Effective Therapy For Muscle Mass Crush Injury In The Rat Surgical Treatment Today Structures of 6 metabolites determined by high-performance fluid chromatography-tandem mass spectrometry in rat plasma, bile, urine, and feces complying with a solitary intramuscular management of 100 µg/ 300 μCi/ kg of [3H] BPC157. In the previously mentioned studies, we defined the pharmacokinetic profile of model BPC157 utilizing high-performance fluid chromatography (HPLC) in rats and pets. Next off, we evaluated the excretion, metabolic rate, and cells circulation of BPC157 in rats after a single IM shot of 100 µg/ 300 μCi/ kg [3H] BPC157. [3H] BPC157 was well endured by all rats, and no aesthetic indications of poisoning were observed. Prolines of BPC157 were classified with [3H] and the framework of [3H] -classified BPC157 is received Number 3A. The concerns of the FDA concerning BPC 157 primarily entail security factors to consider and the absence of detailed clinical trials.Illuminating The Peptide's Device Of Action Within Systems
Bpc 157 Peptide Bpc 157 Review, Side Effects, Dosage, Cycles, Before And After Results - Outlook India
Bpc 157 Peptide Bpc 157 Review, Side Effects, Dosage, Cycles, Before And After Results.
Posted: Tue, 08 Aug 2023 07:00:00 GMT [source]
- Of note, pylorus sphincter failing was believed to reflect reduced esophageal sphincter failure [17,18,20-23]
- These outcomes suggest that urinary excretion is the dominant route of elimination adhering to IM administration of BPC157.
- In the combined pee samples accumulated from 0 to 8 h, the material of [3H] proline (M1), the main metabolite, was greater, accounting for 13.9% (woman) and 11.7% (man) of the overall radioactivity.
- Blood samples were collected at the matching time points before (0 h) and within 6 h of a single administration.
Brain-gut Axis And Pentadecapeptide Bpc 157: Theoretical And Useful Ramifications
We focused on the application of the secure stomach pentadecapeptide BPC 157 [1,2,3,4,5,6,7,8,9,10,11] to enhance the results of spinal cord injury in rats. The theory of cell biology in injury recovery stressed that endothelial cells, fibroblasts, and keratinocytes may contribute to the proliferation stage in the injury healing procedure. To confirm the theory, the MTT assay and cell cycle distribution were utilized to examine the effect of BPC-157 on cell proliferation. Previous studies have found that BPC-157 did not apply a direct result in terms of increasing the cell proliferation of cultured ligament fibroblasts,42 however our outcomes recommended that BPC-157 regulates the cell stability and affects HUVEC cell cycle leave in G0/G1 stage. To analyze the effect of BPC-157 on angiogenesis in vitro, tube formation assay was carried out as defined formerly.28 In this assay, we utilized 2 study procedures. In the very first procedure, development factor-reduced matrigel was pipetted right into prechilled 24-well plates (150 mL matrigel per well) and polymerized for 45 mins at 37 ° C. This was seen before with vessel occlusion (Vukojevic et al., 2018; Gojkovic et al., 2020; Kolovrat et al., 2020; Gojkovic et al., 2021a; Knezevic et al., 2021a; Knezevic et al., 2021a; Knezevic et al., 2021b), alcohol and lithium drunkenness (Gojkovic et al., 2021b; Strbe et al., 2021), and https://s3.us-east-1.wasabisys.com/2udlbbfu4jfp72izc/Biosimilars-development/products/advantages-threats-of-peptide-therapeutics-for-physical-mental.html abdominal aorta anastomosis (Hrelec et al., 2009). The effect took place peripherally (i.e., the biggest apoplexy initially (i.e., 25 mmHg) appeared just in the hepatic veins, looking like the presentation of Budd-- Chiari disorder (Gojkovic et al., 2020)), and centrally (premium sagittal sinus). Abrogated thrombosis, both peripherally and centrally (Vukojevic et al., 2018; Gojkovic et al., 2020; Kolovrat et al., 2020; Gojkovic et al., 2021a; Knezevic et al., 2021a; Knezevic et al., 2021a; Gojkovic et al., 2021b; Knezevic et al., 2021b), indicates that tension was obviously prevented, or at the very least noticeably reduced. Launching the molecular knowledge of BPC-157's influence, its intricate interaction with physical systems looks like an interwoven series of signals and responses. The peptide perfectly slips into the complex mobile network, starting a series of events that speaks with the body's very own language of repair service. To assess the impact of BPC-157 on intracellular signal transduction, the phosphorylation degrees of ERK1/2, JNK, and p38 mitogen-activated healthy protein kinase (MAPK) were analyzed in HUVECs. Outcomes showed that BPC-157 had a dosage-dependent impact on the phosphorylation of ERK1/2 in HUVECs (Figure 6). Beyond the scientific and governing conversations, there's likewise an argument about prospective outside influences on the FDA's decision. There's a huge question mark over just how much influence the large drug companies carry the FDA's decisions. Some people assume that these companies might push the FDA to state no to treatments like BPC 157, particularly if these new therapies can take on their very own products. The FDA claims they only make their decisions based upon strong science and what's finest for every person's wellness. The peptide's interaction with the body is a dancing of accuracy, encouraging cells to discard sleepiness for activity. By rejuvenating the signaling pathways that moderate growth and repair work, BPC-157 imparts a passion for recovery at the foundational degree of organic structure. Extremely, BPC-157 bids capillary to unfurl their network a lot more rapidly, therefore nurturing harmed areas with a rejuvenating circulation. This angiogenic effect moves a cascade of repair, breathing life into cells that formerly rotted in recovery's slow-moving embrace. The detailed ballet of mobile regrowth and fixing discovers a formidable companion in BPC-157, a peptide whose healing touch whispers guarantees of swifter recuperation and restoration.As we turn our interest to the material's regenerative impacts on different tissue kinds, it comes to be clear that understanding its interaction at the cellular degree could transform our approach to healing. Realizing the subtleties of how BPC-157 boosts all-natural healing procedures beckons a much deeper gratitude for the body's inherent durability and capability for self-healing.Is BPC 157 great for heart health and wellness?
In heart disturbances, stable gastric pentadecapeptide BPC 157 personal treatment effects combine the treatment of coronary infarction, cardiac arrest, lung hypertension arrhythmias, and thrombosis prevention and reversal.

Social Links