Restricted Listing World Anti Doping Company
7 Skin Cancer Cells Myths Debunked Fred Hutchinson Cancer Facility
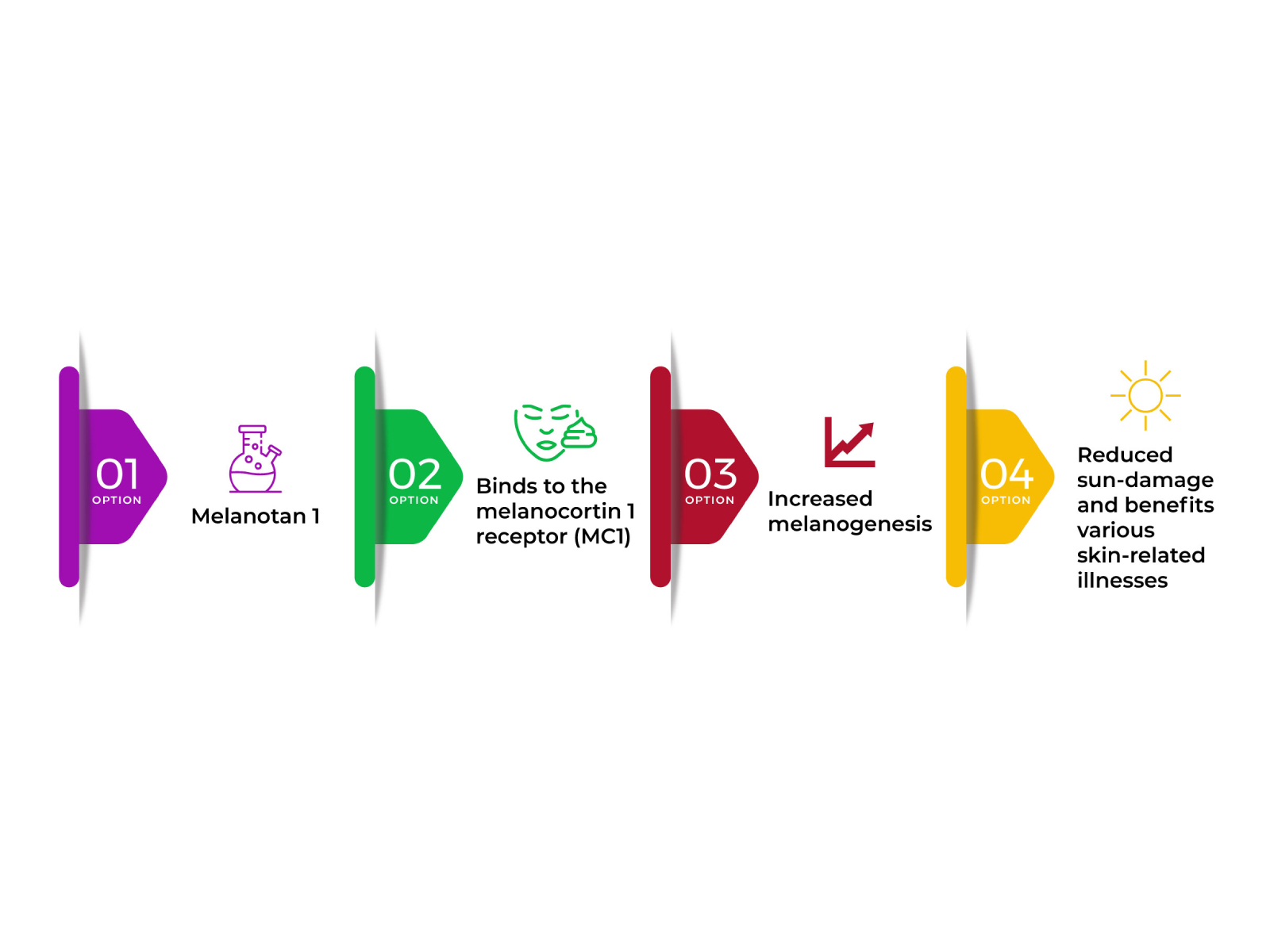
They offer multiple features, from muscular tissue gain, boosting lean muscular tissue mass, and weight loss to increasing recuperation. Manufacturing of eumelanin instead of pheomelanin by melanocytes, thus boosts pigmentation of the skin and hence supplies photoprotection versus hazardous UV radiation from the sunlight. This is the desired restorative benefit from the medical administration of afamelanotide. Production of melanin is usually stimulated by direct exposure to the ultraviolet (UV) radiation. part of sunshine.
- The development of nasal spray tans comes as rates of melanoma have actually rapidly raised in recent years, according to the American Cancer Society.
- MTII was infused ip as opposed to icv because of possible confounding impacts that would arise from intracranial cannulation in suckling pups.
- Background labeling, established using the exact same sampling box over an adjacent region that contained no NPY genetics expression, was deducted from this dimension.
- Depending on exactly how dark you desire your tan, your outcomes can last anywhere from 7 to 10 days.
Melanotan Recommendations
In fact, throughout growth, NPY is shared in a variety of hypothalamic regions that typically do disappoint expression in grown-up rats (8, 9). In the grown-up rat hypothalamus, NPY is expressed generally in the ARH with an added reduced level of expression in the main small area of the DMH (DMHp). In addition to these regions, throughout development, there is a distinct, transient expression of NPY in the noncompact zone of the DMH (DMHnc), the PFR, the LHA, and the PVH (8, 9).
Spray Tan
Skin cancers "can have substantial effect also if they do not kill someone in regards to the devastation they cause," he stated. Several Americans are deficient in vitamin D, and specific teams-- like older adults, overweight individuals and individuals with dark skin-- go to greater danger of a deficiency. And it's true that UV direct exposure without the protection of sunscreen triggers your skin to generate vitamin D. Vitamin D is essential for advertising strong, healthy and balanced bones and has even been linked to lower threats of certain sorts of cancer. After UV damage, your skin shields itself by creating melanin-- the pigment in charge of brownish and black skin tones.
C Enhanced Muscular Tissue Recuperation And Decreased Muscle Mass Discomfort
This puts these steroid customers in jeopardy for obtaining life threatening viral infections, such as HIV and liver disease B and C. 76 In addition, animal designs suggest that anabolic steroids suppress the body immune system,77 which can aggravate infections. Afamelanotide has been shown through Phase III research studies to reduce phototoxic responses and recuperation time in individuals with erythropoietic protoporphyria. Afamelanotide was authorized for therapy of erythropoietic protoporphyria by the European Medicines Agency (EMA) in 2016 and by the FDA in the United States in October 2019. It minimizes discomfort and permits nearly all people to be outdoors for longer durations than formerly. Nonetheless it has no result on the number or period of phototoxic responses.